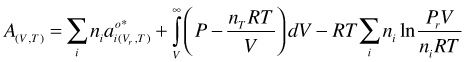Fluid mixtures. As in for mixtures we get:
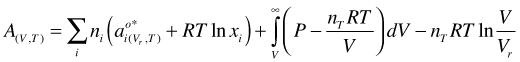
(50)
As for the pure fluid, in the following some transformations of Equation are performed, which have the purpose of obtaining an expression for µi as a function of P and T, so the derived values are compatible with thermodynamic properties with other phase components, e.g. solids. With Vr for an hypothetical ideal gas mixture at reference conditions
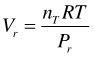
(51)
we get:
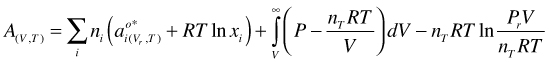
(52)
Incorporation of the ideal mixing entropy into the last term of Equation (52)
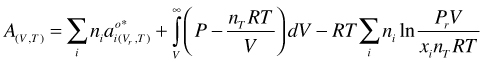
(53)
and replacing the mole fraction xi by its definition (ni /nT) we get: