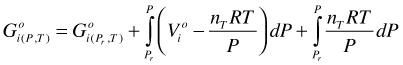P and T as state variables
First we consider P and T as the state variables. In that case G is the appropriate state function. G as a function of P at constant T is calculated by integrating Equation (4). The integration constant in Equation (7) is at the respective reference pressure Pr and any T. The usual choice of Pr is 0.1 MPa. For any real system we have:
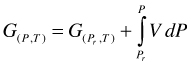
(7)
Pure fluids. For the moment, the standard state of a fluid is the real pure fluid phase component. Pure phase components are generally designated by o. We will change the standard state for the fluid later, however. For such a real pure fluid i in its standard state, Equation (7) becomes:
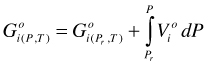
(8)
Now the first rearrangement of Equation uses the ideal gas law, where nT is the total number of moles (![]() ),
),
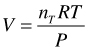
(9)
by expanding (e.g. Gillespie 1925) the argument in the integral of Equation