The container enclosing the substances as defined above is usually referenced as a chemical system. In contrast to the definition above the walls of such a system do not necessarily to be stiff or impermeable for energy transfer or chemical flux. The following important systems can be distinguished (Figure 2):
- opens system: allows energy as well as chemical transfer into and from the system
- closed system: allows only energy transfer
- isolated system: no transfer at all
- adiabatic system: only transfer of work
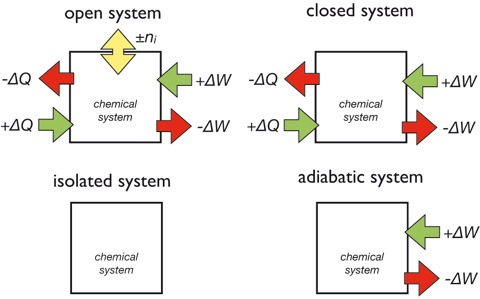
Figure 2: The definitions of the open, closed, isolated and adiabatic system.
Therefore the definition of the container at the beginning of this chapter is that of a closed system. Because the mechanical work W as treated here is volume work (compression or expansion of the system, see below) the walls for which the transfer of W is allowed are not rigid.