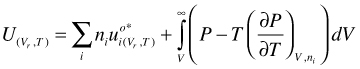U as function of V and T. Integration of gives:
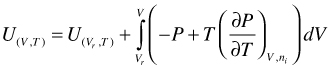
(95)
For an ideal gas:
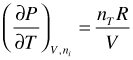
(96)
As a result, the integral in (95) is zero for an ideal gas and therefore U is in this case not a function of V. This behavior is understandable, because there is no intermolecular interaction between particles. Expansion of Equation (95), and splitting the term into an ideal gas and a deviation part like in Equation (10) is, therefore, not necessary
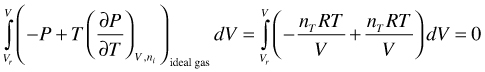
(97)
However, expansion of the integration boundaries is again useful
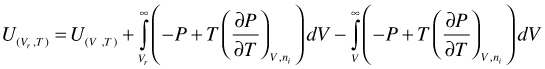
(98)
Combination of the first two terms of the right hand side of Equation (98) yields U for a hypothetical ideal gas. In addition, for an ideal gas mixture, there is no excess U
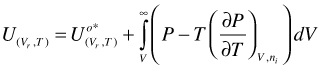
(99)